mRNA Therapeutics CDMO Market Grows at 11.37% CAGR to Hit USD 13.63 Bn by 2034
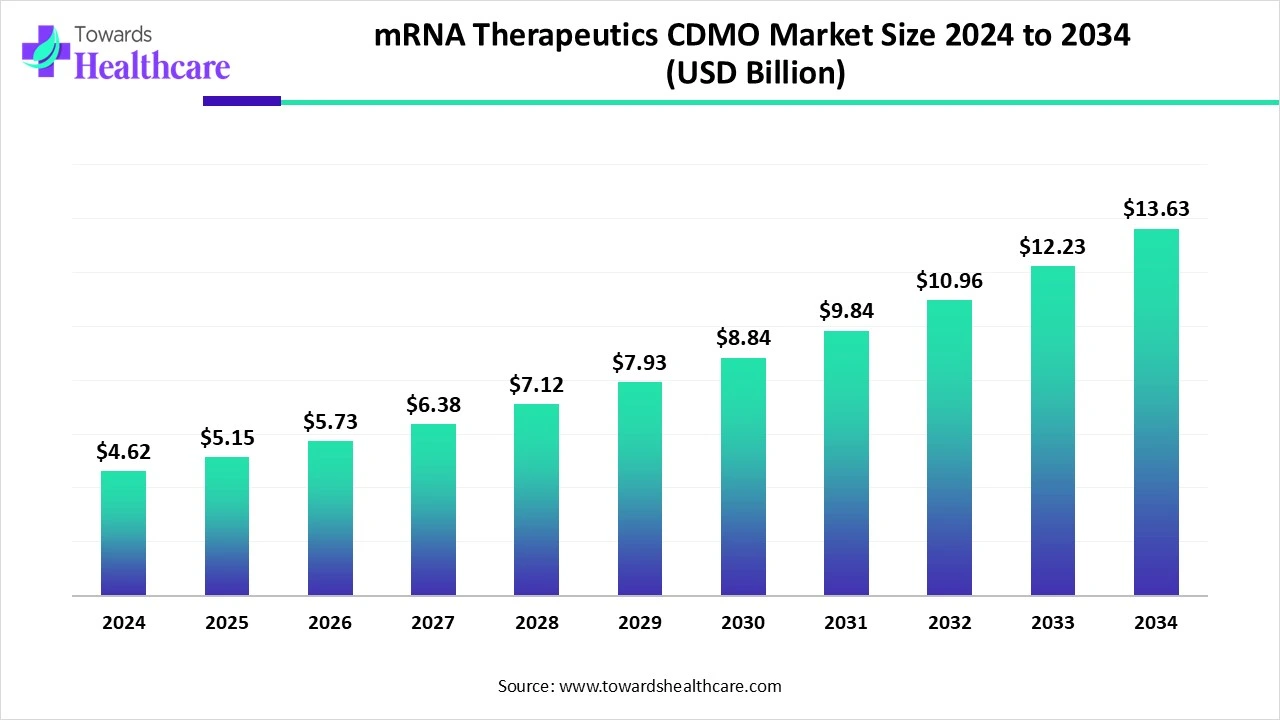
The global mRNA therapeutics CDMO market size is calculated at USD 5.15 billion in 2025 and is expected to reach around USD 13.63 billion by 2034, growing at a CAGR of 11.37% for the forecasted period.
Ottawa, Oct. 15, 2025 (GLOBE NEWSWIRE) -- The global mRNA therapeutics CDMO market size was valued at USD 4.62 billion in 2024 and is predicted to hit around USD 13.63 billion by 2034, rising at a 11.37% CAGR, a study published by Towards Healthcare a sister firm of Precedence Research.
The Complete Study is Now Available for Immediate Access | Download the Sample Pages of this Report @ https://www.towardshealthcare.com/download-sample/5831
Key Takeaways
- The mRNA therapeutics CDMO industry poised to reach USD 4.62 billion in 2024.
- Forecasted to grow to USD 13.63 billion by 2034.
- Expected to maintain a CAGR of 11.37% from 2025 to 2034
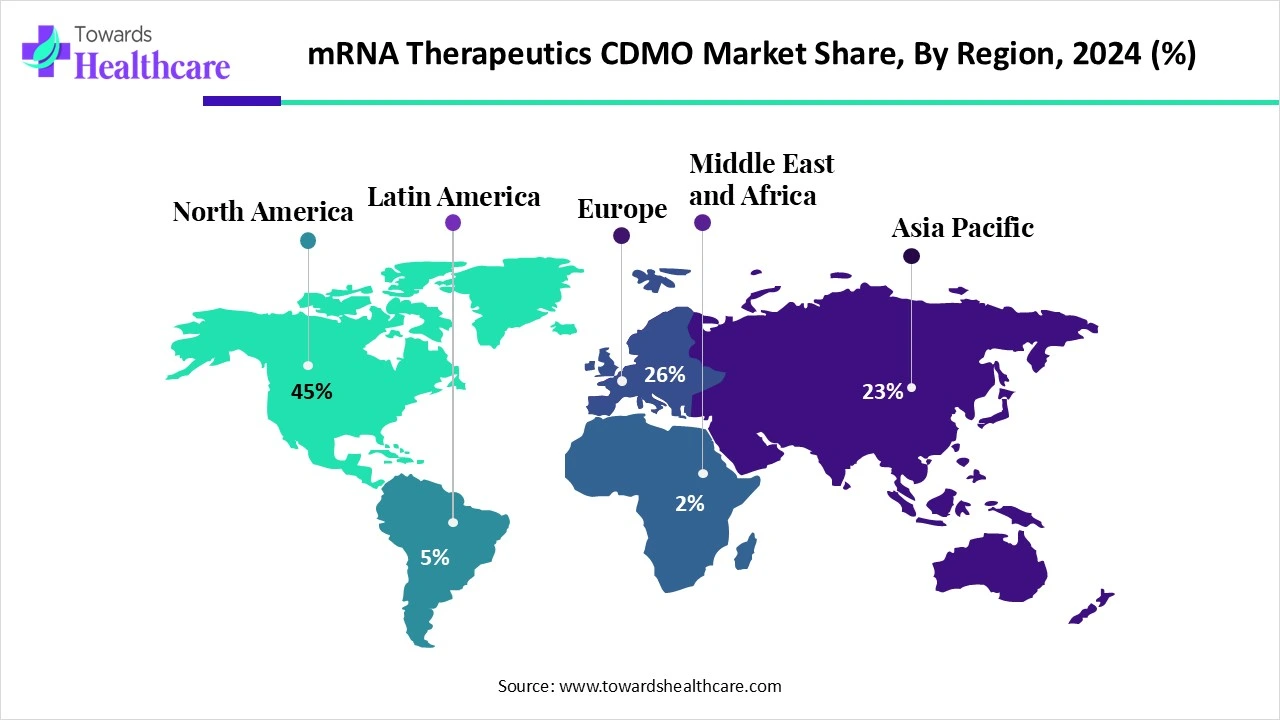
- North America registered dominance in the market by 45% in 2024.
- Europe is expected to grow at the fastest CAGR during 2025-2034.
- By service type, the mRNA drug substance manufacturing segment led the mRNA therapeutics CDMO market in 2024.
- By service type, the end-to-end CDMO services segment is expected to witness the fastest growth during the forecast period.
- By application, the infectious diseases segment dominated the market in 2024.
- By application, the oncology segment is expected to be the fastest-growing in the predicted timeframe.
- By scale of operation, the commercial manufacturing segment was dominant in the market in 2024.
- By scale of operation, the clinical manufacturing segment is expected to register rapid expansion during 2025-2034.
- By end-user, the biopharmaceutical & biotechnology companies segment led the market in 2024.
- By end-user, the personalized medicine companies segment is expected to grow at the fastest CAGR in the studied years.
What is the mRNA Therapeutics CDMO?
The global mRNA therapeutics CDMO market is offering specialized services for the research, development, and production of messenger RNA-based drugs, like vaccines and therapies for genetic diseases and cancer. The market is propelled by the ongoing growth of mRNA applications beyond vaccines into oncology and rare diseases, breakthroughs in production processes, and the rising complexity of mRNA-based therapies. Nowadays, the market is emphasizing improvements in delivery systems through enhanced lipid nanoparticle (LNP) technology, accelerating production efficiency with AI and continuous manufacturing methods.
You can place an order or ask any questions, please feel free to contact us at sales@towardshealthcare.com
What are the Key Drivers in the mRNA Therapeutics CDMO Market?
Numerous pharmaceutical and biotech companies are highly outsourcing the complex, specialized manufacturing processes for mRNA, which fuels demand for CDMO expertise. Moreover, the burgeoning clinical pipeline of mRNA drug candidates is enhancing the demand for these specialized facilities. Alongside, the acceleration of self-amplifying mRNA (saRNA) and circular RNA (circRNA) develops demand for specialized production techniques from CDMOs.
What are the Major Drifts in the mRNA Therapeutics CDMO Market?
The widespread companies are investing and collaborating with other major players for the future development of mRNA-based therapies, expansion of production facilities, and innovations in mRNA.
- In April 2025, Wacker Biotech, a CDMO, collaborated with Boston-based RNAV8 Bio, focused on transforming the development and production of mRNA-based advanced therapies for the biopharma industry.
- In November 2024, Japan-based Meiji Seika Pharma Co. invested in ARCALIS, Inc. to expand production of mRNA vaccines in Japan.
What is the Emerging Challenge in the Market?
The global mRNA therapeutics CDMO market is facing complex regulatory barriers, conflicts in scaling up manufacturing. Also, it is difficult to maintain quality and supply chain vulnerabilities for crucial raw materials, such as nucleotides and lipid nanoparticles.
Regional Analysis
How did North America Dominate the Market in 2024?
In 2024, North America accounted for the biggest revenue share by 45% of the mRNA therapeutics CDMO market. Key drivers are the raised R&D investment, as well as the expansion of the outsourcing trend, for those biotech & pharma companies that are dependent on specialized CDMOs for manufacturing. The latest efforts from Lonza, venture capital funding for startups, including Strand Therapeutics, and novel alliances led by government support, like the BARDA award to Moderna for influenza preparedness, are also fostering the overall progression.
For instance,
- In June 2025, Ethris GmbH, a clinical-stage biotechnology company, collaborated with Thermo Fisher Scientific to offer a fully integrated mRNA solution to biopharmaceutical developers.
Download the single region market report @ https://www.towardshealthcare.com/checkout/5831
Why did Europe Grow Notably in the Market in 2024?
In the upcoming era, Europe is anticipated to witness rapid expansion in the mRNA therapeutics CDMO market. The market is fueled by a rise in advances in manufacturing technology for scalability and flexibility, robust government and regulatory support in regions, primarily Germany and the UK. In June 2025, the World Health Organization explored the next phase of the mRNA Technology Transfer Programme, with a focus on supporting manufacturers in lower- and middle-income countries to expand viable mRNA production, in which many European governments and the EU are leveraging initiatives. (Source- WHO)
Latest Updates in mRNA Therapeutics CDMO Market
| US FDA | Updated warning labels for mRNA COVID-19 vaccines from Pfizer and Moderna related to the challenge of myocarditis (inflammation of the heart muscle) and pericarditis (inflammation of the lining of the heart). |
| EMA | Introduced a public consultation on a draft guideline that covers the quality aspects of mRNA vaccines for infectious diseases. |
Become a valued research partner with us - https://www.towardshealthcare.com/schedule-meeting
Segmental Insights
By service type analysis
Which Service Type Dominated the mRNA Therapeutics CDMO Market in 2024?
The mRNA drug substance manufacturing segment captured a major share of the market in 2024. The combination of factors, like the growing R&D investment, application in cancer, & rare diseases, as well as the increasing government initiatives in mRNA projects, is assisting the overall market expansion. Moreover, expanded CDMOs are offering more flexible, multi-product provision in accommodating the various needs of mRNA innovators. It usually encompasses template generation, in vitro transcription, purification, characterization, and scaling up.
Whereas the end-to-end CDMO services segment will expand rapidly. Currently, these services are focusing on breakthroughs in digital integration, with CDMOs adopting AI-powered quality control and expanding into novel therapeutic areas, including oncology and rare diseases. As well as they are also widely involved in more investment in lipid nanoparticle (LNP) production and continuous manufacturing to boost yields and reproducibility.
By application analysis
How did the Infectious Diseases Segment Lead the Market in 2024?
The infectious diseases segment held a dominant share of the mRNA therapeutics CDMO market in 2024. Especially, the globe is fostering efforts in the development of mRNA-based vaccines for viruses, such as Zika, Nipah, and mpox. CDMOs are widely involved in expanding therapies for herpes simplex virus (HSV), Epstein-Barr virus (EBV), and cytomegalovirus (CMV). Alongside, they are stepping into innovations in stabilization, delivery systems, and advanced manufacturing technologies.
Although the oncology segment is estimated to register rapid expansion during 2025-2034. Globally rising cancer cases and wider demand for precision medicine are supporting the adoption of mRNA solutions in the developed and emerging CDMOs. Additionally, CDMOs are shifting towards more agile production methods, applying modular and single-use systems. This further enables quicker adjustments and faster transitions from clinical-scale to commercial-scale production.
Get the latest insights on life science industry segmentation with our Annual Membership: https://www.towardshealthcare.com/get-an-annual-membership
By scale of operation analysis
What Made the Commercial Manufacturing Segment Dominant in the Market in 2024?
In the mRNA therapeutics CDMO market, the commercial manufacturing segment held a major share in 2024. Ongoing investments in mRNA research, progressing clinical pipeline, and expanding strategic collaboration are bolstering the segment growth. Currently, CDMOs are leveraging diverse AI-enabled solutions, along with facilitating complete, one-stop solutions from the initial stages of plasmid DNA synthesis to the final mRNA product and lipid nanoparticle (LNP) encapsulation.
However, the clinical manufacturing segment will expand rapidly in the coming era. Continuous advances in mRNA synthesis and next-generation technologies, including self-amplifying and circular RNA, are imposing demand for specialized CDMO facilities. CDMOs are further emphasizing the growth of GMP facilities, collaborations for process enhancement, and novel approaches in lipid nanoparticle (LNP) delivery.
By end-user analysis
Why did the Biopharmaceutical & Biotechnology Companies Segment Lead the Market in 2024?
The biopharmaceutical & biotechnology companies segment held the largest share of the mRNA therapeutics CDMO market in 2024. Major companies, like Thermo Fisher Scientific, Lonza, and Merck, are providing mRNA development and manufacturing, comprising products and services for gene synthesis, in vitro transcription, and purification. Also, exploring extensive capabilities in mammalian cell culture and advanced therapies, with affordable, large-scale solutions in production.
On the other hand, the personalized medicine companies segment is anticipated to witness the fastest growth. These companies are mainly focusing on the personalization of mRNA-based immunotherapies for cancer, also offering custom mRNA, modified nucleotides, and proprietary capping technology, necessary components for customized medicine applications. BioNTech, eTheRNA, and TriLink BioTechnologies are specifically involved in the respective approaches.
Browse More Insights of Towards Healthcare:
The global vaccine adjuvant market was valued at USD 702.4 million in 2024 and is expected to reach USD 747.6 million in 2025, with projections pointing to nearly USD 1,303.4 million by 2034, growing at a CAGR of 6.43% over the forecast period.
The miRNA tools and services market stood at USD 417.69 million in 2024, rising to USD 498.34 million in 2025, and is anticipated to reach approximately USD 2,399.94 million by 2034, expanding at a CAGR of 19.34% between 2025 and 2034.
The NGS-based RNA sequencing market was valued at USD 3.74 billion in 2024 and is projected to grow to USD 4.49 billion in 2025, reaching around USD 23.52 billion by 2034, with a CAGR of 20.1% over the forecast timeline.
The RNA-based therapeutics market was estimated at USD 6.83 billion in 2023 and is expected to grow to USD 40.81 billion by 2034, at a CAGR of 17.64% from 2024 to 2034.
The miRNA sequencing and assay market reached USD 391.73 million in 2024, is set to hit USD 443.95 million in 2025, and is projected to achieve USD 1,369.11 million by 2034, growing at a CAGR of 13.33% during 2024–2034.
The global microRNA market was valued at USD 1.61 billion in 2023 and is expected to reach USD 6.11 billion by 2034, growing at a CAGR of 12.89% from 2024 to 2034.
The gRNA market was estimated at USD 499.21 million in 2023 and is projected to expand to USD 3,296.45 million by 2034, rising at a CAGR of 18.72% from 2024 to 2034.
The antisense and RNAi therapeutics market is forecast to increase from USD 7.38 billion in 2025 to USD 35.63 billion by 2034, growing at a CAGR of 19.12% over the forecast period.
The RNAi therapeutics market is projected to grow from USD 1.47 billion in 2025 to USD 5.11 billion by 2034, at a CAGR of 14.9% between 2025 and 2034.
Finally, the liquid biopsy tube market was valued at USD 1.34 billion in 2024, expected to reach USD 1.54 billion in 2025, and is anticipated to climb to USD 5.32 billion by 2034, expanding at a CAGR of 14.85% from 2025 to 2034.
Major Developments in the mRNA Therapeutics CDMO Market
- In September 2025, the UK inaugurated the Moderna mRNA Vaccine Centre in Oxfordshire and launched a £50 million life-sciences fund to expand pandemic-scale vaccine manufacturing and escalate R&D investment in the UK’s life-sciences sector.
- In September 2025, Elegen, a company in cell-free DNA manufacturing, officially introduced ENFINIA IVT Ready DNA, created for immediate use in in vitro transcription (IVT), with minimal time required for the development of mRNA-based therapeutics by weeks.
- In January 2025, Esphera SynBio, a pre-clinical stage synthetic biology company, unveiled a new project focused on boosting the efficacy of mRNA vaccines.
mRNA Therapeutics CDMO Market Key Players List
- CordenPharma
- Lonza Group AG
- Samsung Biologics
- Catalent Inc.
- Wacker Biotech GmbH
- Aldevron (a Danaher company)
- Thermo Fisher Scientific (Patheon)
- BioNTech Manufacturing Services
- Moderna Manufacturing Partnerships
- Emergent BioSolutions
- Evonik Industries AG
- Rentschler Biopharma
- Recipharm AB
- TriLink BioTechnologies (Maravai LifeSciences)
- Acuitas Therapeutics (LNP tech provider)
- eTheRNA Manufacturing
- IDT (Integrated DNA Technologies, part of Danaher)
- WuXi Biologics / WuXi STA
- AGC Biologics
- MilliporeSigma (Merck KGaA CDMO division)
Download the Competitive Landscape market report @ https://www.towardshealthcare.com/checkout/5831
Segments Covered in the Report
By Service Type
- mRNA Drug Substance Manufacturing (IVT)
- End-to-End CDMO Services
- Integrated drug development from plasmid to vial
- Plasmid DNA Production
- LNP Formulation & Encapsulation
- Fill-Finish & Aseptic Packaging
- Analytical & Quality Control Testing
- Regulatory & CMC Support
By Application
- Infectious Diseases
- COVID-19
- Influenza
- RSV, Zika, CMV
- Oncology (Cancer Vaccines & Immunotherapies)
- Rare Genetic Disorders
- Autoimmune Diseases
- Cardiovascular & Metabolic Diseases
- Personalized Cancer Vaccines
By Scale of Operation
- Commercial Manufacturing
- Clinical Manufacturing
By End-User
- Biopharmaceutical & Biotechnology Companies
- Personalized Medicine Companies
- Academic & Research Institutions
- Government & Public Health Agencies (e.g., CEPI, BARDA)
By Region
- North America
- U.S.
- Canada
- Asia Pacific
- China
- Japan
- India
- South Korea
- Thailand
- Europe
- Germany
- UK
- France
- Italy
- Spain
- Sweden
- Denmark
- Norway
- Latin America
- Brazil
- Mexico
- Argentina
- Middle East and Africa (MEA)
- South Africa
- UAE
- Saudi Arabia
- Kuwait
Immediate Delivery Available | Buy This Premium Research @ https://www.towardshealthcare.com/checkout/5831
Access our exclusive, data-rich dashboard dedicated to the healthcare market - built specifically for decision-makers, strategists, and industry leaders. The dashboard features comprehensive statistical data, segment-wise market breakdowns, regional performance shares, detailed company profiles, annual updates, and much more. From market sizing to competitive intelligence, this powerful tool is one-stop solution to your gateway.
Access the Dashboard: https://www.towardshealthcare.com/access-dashboard
About Us
Towards Healthcare is a leading global provider of technological solutions, clinical research services, and advanced analytics, with a strong emphasis on life science research. Dedicated to advancing innovation in the life sciences sector, we build strategic partnerships that generate actionable insights and transformative breakthroughs. As a global strategy consulting firm, we empower life science leaders to gain a competitive edge, drive research excellence, and accelerate sustainable growth.
You can place an order or ask any questions, please feel free to contact us at sales@towardshealthcare.com
Europe Region: +44 778 256 0738
North America Region: +1 8044 4193 44
APAC Region: +91 9356 9282 04
Web: https://www.towardshealthcare.com
Our Trusted Data Partners
Precedence Research | Statifacts | Towards Packaging | Towards Automotive | Towards Food and Beverages | Towards Chemical and Materials | Towards Consumer Goods | Towards Dental | Towards EV Solutions | Nova One Advisor | Healthcare Webwire | Packaging Webwire | Automotive Webwire
Find us on social platforms: LinkedIn | Twitter | Instagram | Medium | Pinterest

Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.

